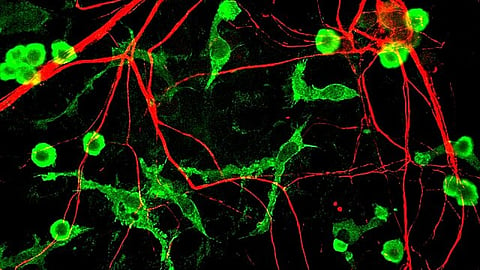
“We want to see how these cells, particularly those without the two genes, impact Alzheimer 's-related phenotypes in both dish and mouse models. We predict that in mice, microglia without BHLHE40 and 41 will clear away beta-amyloid plaques more effectively than control microglia that have normal levels of BHLHE40 and 41. Also, we're exploring how lack of these genes in the brain immune cells affect other types of cells in the brain such as neurons and astrocytes,” says Dr. Goate.
The paper is titled “BHLHE40/41 regulate microglia and peripheral macrophage responses associated with Alzheimer’s disease and other disorders of lipid-rich tissues.”
The remaining authors of the paper, all with Icahn Mount Sinai except where indicated, are: Anna Podlesny-Drabiniok, PhD; Gloriia Novikova, PhD; Yiyuan Liu, PhD; Josefine Dunst, PhD (Anocca in Sweden); Rose Temizer, PhD candidate (National Institute of Mental Health); Samuele Marro, PhD; Taras Kreslavskiy, PhD (Karolinska Institute); and Edoardo Marcora, PhD.
The study was made possible by funding from various sources, including NIH grants (RF1AG054011, U01AG058635, R56AG081417, U01AG066757, NHLBI R01HL153712, S10OD026880 and S10OD030463) and support from The JPB Foundation and BrightFocus Foundation (A2021014F), the New York State Department of Health stem cell biology fellowship (NYSTEM- C32561GG), America Heart Association (20SFRN35210252), and Graduate Women in Science Fellowship.
(Newswise/NJ)