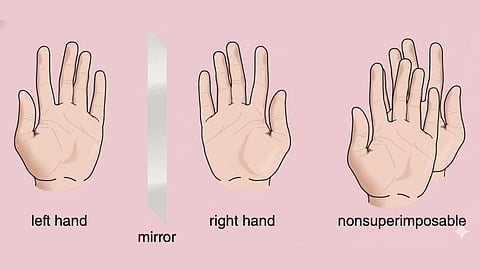
Biological molecules such as enzymes, receptors, and proteins are themselves chiral. Even a small change in orientation can determine whether a molecule fits perfectly, poorly, or not at all.
In a discussion on MedBound Hub regarding chirality of molecules and how they interact with the body, Ketan Laxman Sonawane, Master of Pharmacy in Quality Assurance, explained:
"Our body is highly selective. The receptors and enzymes that interact with medicines are also three-dimensional and “handed.” So, when a drug enters the body, one mirror-image form may fit perfectly into a receptor and produce the desired healing effect."
He further explained, " Its twin, however, may not fit well at all. Sometimes it does nothing. In worse cases, it may even cause unwanted side effects."
The Language of Chirality: R and S