A nationwide voluntary recall has been announced in the U.S. by BRS Analytical Services, citing potential health hazards associated with the affected eye care products. [1]
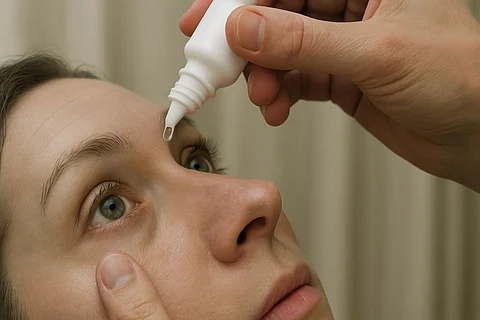
Certain eye care products used as artificial tears, manufactured by the well-known pharmaceutical company AvKARE, were recalled by BRS Analytical Service, LLC following an audit by the FDA that reported faulty manufacturing practices and concerns regarding cGMP (Current Good Manufacturing Practice) violations.
Artificial tears are eye drops commonly used to relieve dry, itchy eyes by keeping them moist and lubricated. A large number of people suffering from ophthalmic conditions rely on these products, and ophthalmologists frequently prescribe them as part of routine eye care.